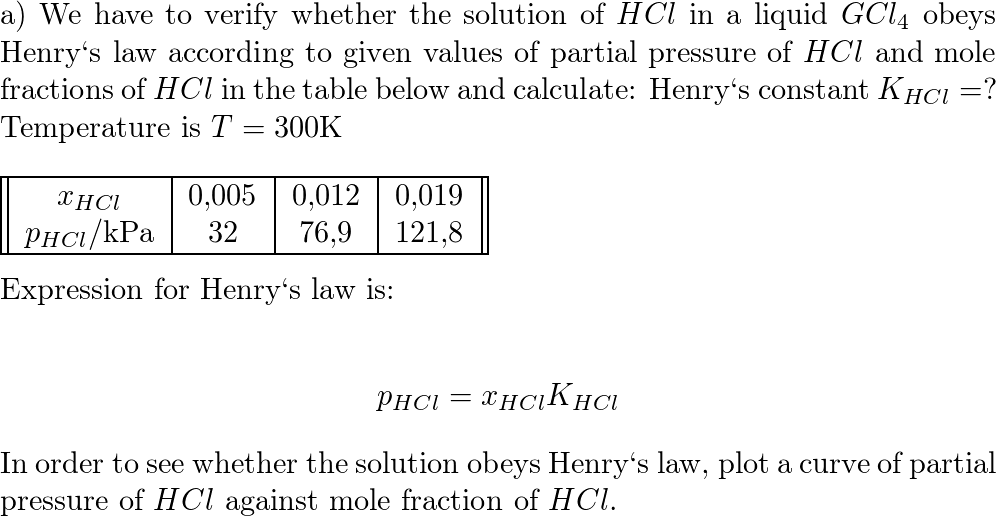The heat of formaiton of HCl at 348 K from the given data will be :1/2 H 2 g +1/2 Cl 2 g → HCl g H 2980= 22060 cal mol 1The

Lecture 1: Introduction and review –Quiz 1 –Website: –Review of acid/base chemistry –Universal features of. - ppt download
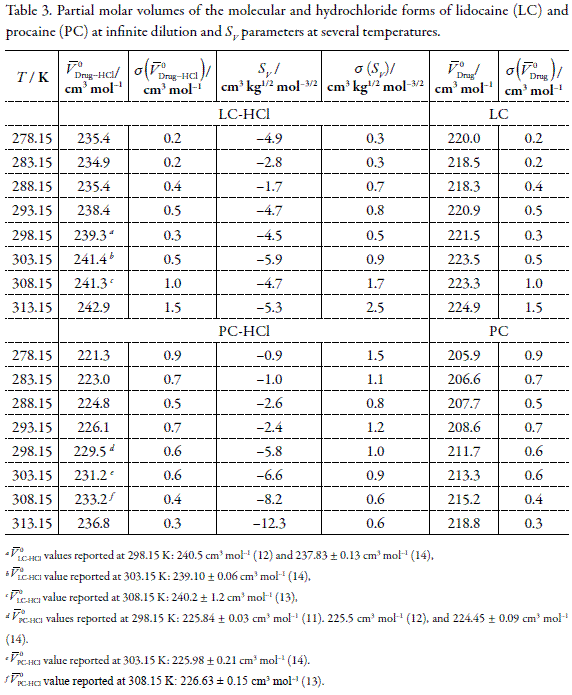
Apparent molar volumes of the anesthetic drugs procaine-HCl and lidocaine- HCl in water at temperatures from 278.15 to 313.15 K

Calculate the work done (J) when 1 mole of zinc dissolves in hydrochloric acid at 273 K in a closed beaker at 300 K [Volume at STP = 22.4 L, rounded up
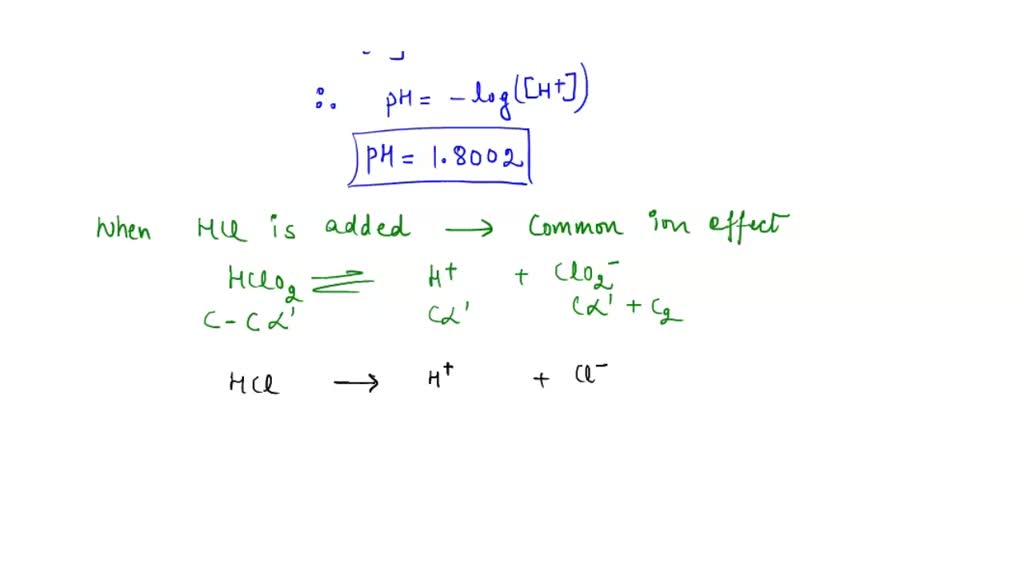
SOLVED: Determine the pH of a solution that is 0.00424 M HCl and 0.0228 M HClO2. The Ka of HClO2 is 1.1×10âˆ'2.

Binding Association Constants (Ka ) and Binding Sites (N) for Three... | Download Scientific Diagram



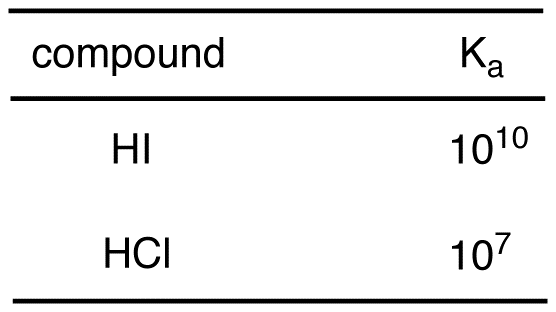


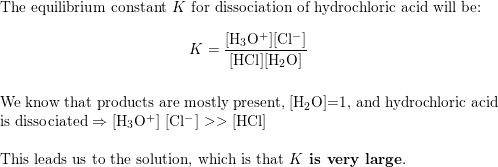

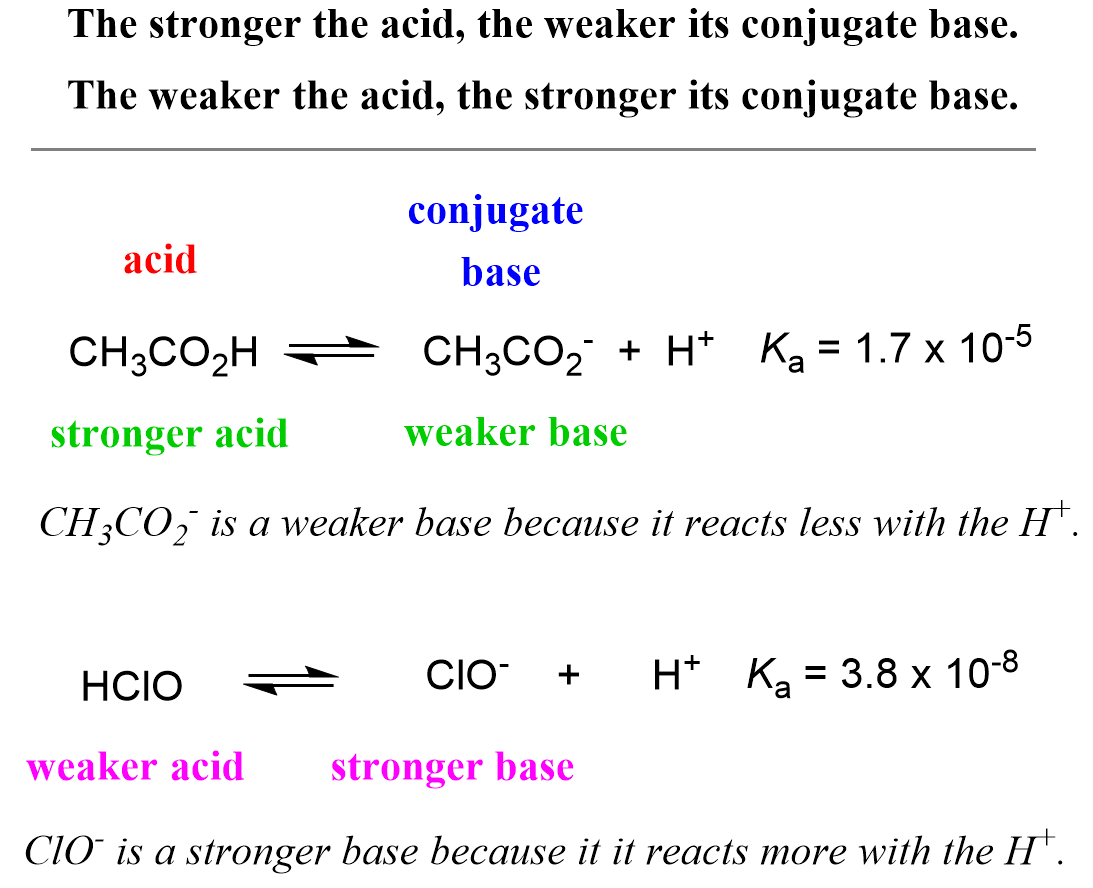
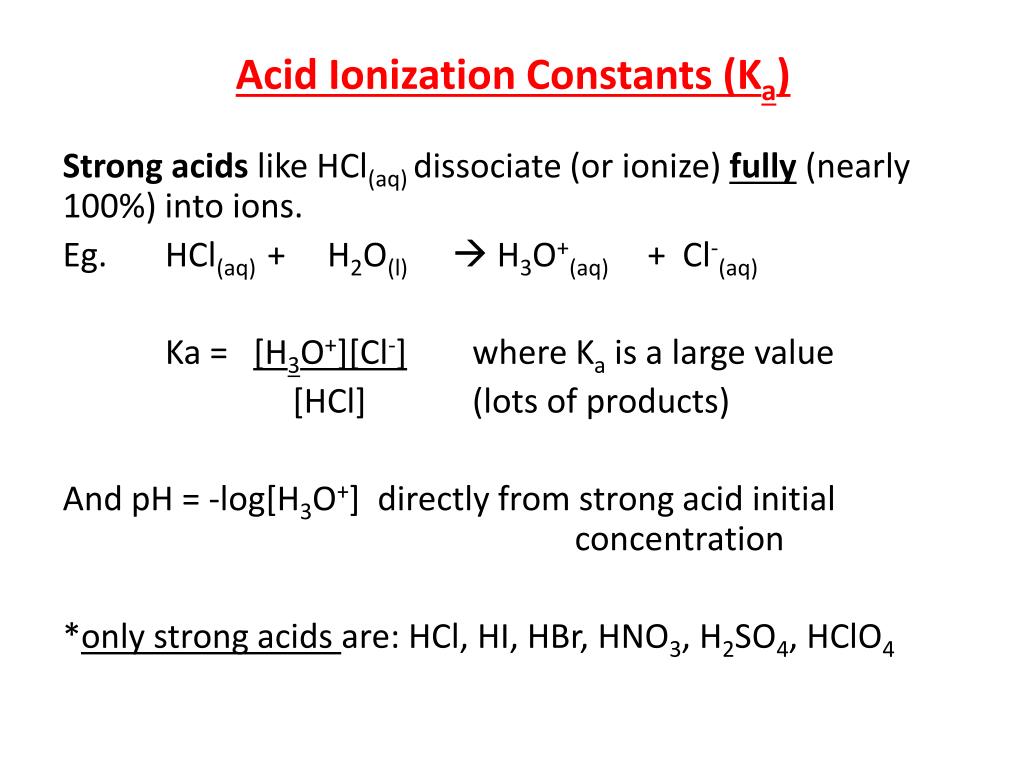

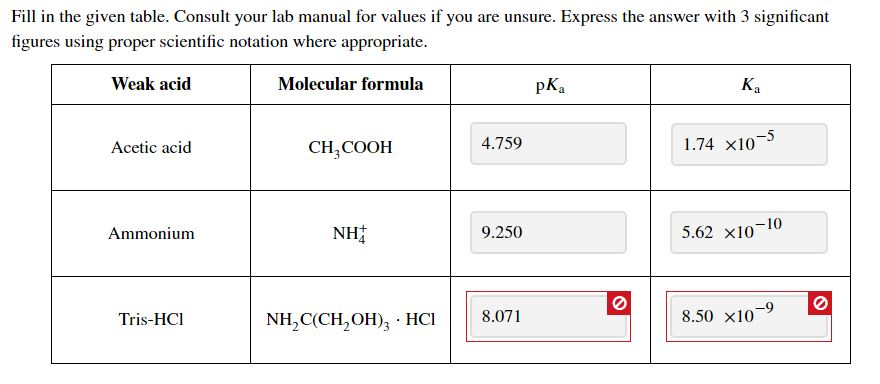

![Solved HCl + H2O → H30+ + CH) = Ka = [H3O+] [C) = 1.3 x 106 | Chegg.com Solved HCl + H2O → H30+ + CH) = Ka = [H3O+] [C) = 1.3 x 106 | Chegg.com](https://media.cheggcdn.com/media/0fe/0fe53724-71a9-4b4c-a2f3-a791d50b9bb2/phpCUvOU0)